A Novel Genome-Edited Hematopoietic Blood Cell System for Cancer (AML) Therapy
用於癌症 (AML) 治療的新型基因組編輯造血細胞系統

- A novel genome-edited hematopoietic blood cell system expressing the hematopoietic transcription factor EKLF(K54R) with change of the SUMOylating site brings new
opportunities of disease therapy and extension of health span / life span;
- In preclinical study, mice expressing the orthologous mutant EKLF (K74R) lived long and healthy with little cancer incident, and mouse primary natural killer cells, NK(K74R) cells displayed resistance of different cancers by in vitro and in vivo;
- Human embryonic stem cell (ESC)-derived NK(K54R) demonstrated robust higher anti-cancer (including leukemia) capability by in vitro and in vivo as well.
- 表達造血轉錄因子 EKLF(K54R) 並改變 SUMOylating 位點的新型基因組編輯造血血細胞系統帶來了新的疾病治療和延長健康/壽命的機會;
- 在臨床前研究中,表達直系同源突變體EKLF(K74R)的小鼠壽命長且健康,癌症事件很少,小鼠原發性自然殺傷細胞NK(K74R)細胞在體外和體內表現出對不同癌症的抵抗力;
- 人胚胎幹細胞 (ESC) 衍生的 NK(K54R) 在體外和體內均表現出強大的更高抗癌(包括白血病)能力。
 Targeted indication
Targeted indication
Cancer/AML(acute myeloid leukemia) therapy:
by human ESC-derived NK(K54R) cells or by EKLF(K54) genome-edited human hematopoietic stem/progenitors (HSPC)
 Status
Status
Pre-clinical studies and MOA identification
 Key features
Key features
- - A blood system expressing the mutant EKLF (K74) allows mice ,and human by implication,to live long and healthy;
- - High anti-cancer capability of specific types of immune cellsexpressing the mutant EKLF factor.
 Market
Market
- The cancer market size exceeded USD 270.5 billion in 2021 and is anticipated to grow at 10.2% CAGR between 2022 and 2028. The global AML treatment market size is USD$ 560M in 2022, and projected to reach over USD$ 10 billion by 2027. About 60-80% of AML adult patients can attain a complete remission with intensive induction chemotherapy; however, all of these patients will relapse within a median of 4-8 months if without additional cytotoxic therapy (P. A. Cassileth et al., J Clin Oncol, 1988; Richard A Larson, 2022, UpToDate, accessed 23 Sep, 2022,). Bone marrow transplantation (BMT) is a method for curing AML patients with poor prognostic factors, but the main risks of BMT are MHC rejection, infection and recurrence. Also, at the present time, NK cell therapy for cancer including AML is still dominated by unmodified NK cells, but the trend of gene-modified NK cell therapy has reached 30%, indicating that the focus of future NK cell therapy will shift to gene/cell therapy.
- - Our pre-clinical studies have shown that mouse Eklf(K74R) as well as human EKLF(K54) genome-edited hematopoietic blood cells have better therapeutic effects than WT, suggesting that it will be clinically superior to unmodified MNC/NK/HSPC cell therapy in the future.
MODE OF ACTION
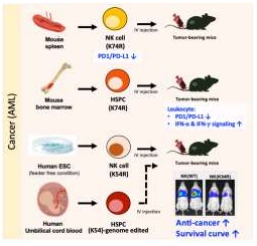
Mouse Eklf (K74R) hematopoietic blood cell system carries high anti-cancer capability due to several characteristics including the low levels of the immune checkpoint proteins (PD-1, PD-L1 and PD-L2) as well as modulation of the leukocyte cellular pathways in the anti-cancer direction. Also, both mouse NK(K74R) and human NK(K54R) cells carry higher anti-cancer ability and they improved the survival rate of tumor-bearing mice.
EXPERIMENTAL RESULTS
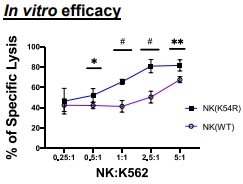
Human NK (K54R) cells have better in vitro cytotoxicity against cancer cells than wild-type NK cells. NK cells were derived from human ESC after 2 months of differentiation process in vitro. The killing rates of K562 (leukemia cells) were determined by the intensity of fluorescence in the medium and normalized with spontaneous background and used positive control as 100%.
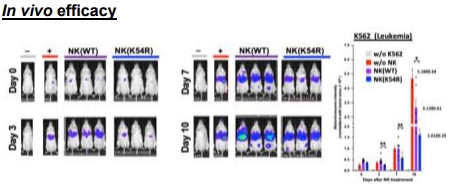
Human NK (K54R) cells have better anti-cancer capability than NK (WT) cells in NSG mice bearing leukemia. K562-Luc cells were injected into WT NSG mice. After 4 days when tumor growth was enough to be observed by IVIS, the mice received ESC-derived NK cells at day-0 by tail vein injection. Note that the NK(K54R) (blue line and bar) has better anti-tumor ability than NK(WT) (purple line and bar)
Eklf (K74R) mice lived with extended lifespan and healthspan;
No obvious side effects on tumor-bearing rodent model after injection of human NK (K54R) or mouse NK(K74R);
Human engineered NK92 cell therapy (CAR-NK) has been used in several clinical trials from phase I/II. The above suggest that MNC transplantation and NK injection are preclinically safe and without toxicity
INTELLECTUAL PROPERTY
SELECTED PUBLICATIONS
Shyu et al. (2022). Advanced Science,9, e2201409.
Hung et al.(2020). International Journal of Molecular Sciences, 21(22): 8448.
BUSINESS OPPORTUNITY
Licensing and/or Collaboration, Sponsored Research
CONTACT
service@biip-dcc.org
